ビジネスワイヤ(バフェットのBerkshire Hathaway group)
ヌークレアス・ソフトウェア、日本におけるパートナーシップと… 2026年01月09日 20時08分
日本の金融エコシステムおよびグローバルなフィンテック協業への長期的なコミットメントを再確認
東京--(BUSINESS WIRE)--(ビジネスワイヤ) --日本ヌークレアス・ソフトウェア株式会社は、日本におけるパートナーシップおよびイノベーションの25周年という重要な節目を迎え、帝国ホテル 東京で開催された特別な集いを通じて、これを祝いました。


当夜は、日本の主要な金融機関の経営幹部、グローバルなフィンテック分野のリーダー、そして長年のパートナーが一堂に会し、日本の金融コミュニティとヌークレアス・ソフトウェアとの関係性の深さを映し出す、格式ある集いとなりました。また、三井住友フィナンシャルグループ(SMFG)、SBI新生銀行、みずほ銀行、トヨタファイナンシャルサービス、日本製鉄(日鉄ソリューションズ株式会社)をはじめとする、日本の金融エコシステムを代表する機関の著名なリーダーの出席により、いっそう意義深いものとなりました。
イベントは、日本の伝統と未来志向のイノベーションが時を超えて調和する姿を象徴する、伝統的な三味線の演奏で幕を開けました。その精神は、ヌークレアス・ソフトウェアにとって、四半世紀にわたりパートナーシップを導いてきたものでもあります。
八城政基氏(元新生銀行会長兼CEO)は、新生銀行が歩んできた再生とレジリエンスの軌跡を振り返り、数十年前に画期的なイノベーションを取り入れたシステム構築の重要性を強調しました。また、ダナンジャヤ・デュイベディ氏(元新生銀行専務執行役員兼CIO)が、巨大なマシンに代えて小型の最新コンピューターを導入し、想定投資額の1/10 で、かつ4〜5倍の速度を実現した点における多大な貢献を強調しました。八城氏の発言は、イノベーションとスピードを重視して価値を提供し、日本の金融セクターに貢献し続けるヌークレアス・ソフトウェアの理念と強く共鳴するものでした。
本イベントの基調講演者を務めた磯和啓雄氏(SMBCグループ執行役専務兼グループCDIO)は、日本、グローバル、そして特にインドにおけるSMBCのデジタル施策について語り、信頼と長期的な目的に根差したイノベーションの重要性を強調しました。また、ヌークレアス・ソフトウェアがインドにおいてSMFGを継続的に支援してきたことにも言及し、共通するエンジニアリングの卓越性と一致したビジョンが、引き続き意義ある価値を創出している点にも触れました。
当夜には、「SBI Shinsei Bank Transformation Journey & What Lies Ahead」をテーマとしたファイアサイドディスカッションも行われ、 岡野道征氏(SBI新生銀行ITスペシャルアドバイザー兼元専務執行役員)、 浅野仁氏(SBI新生銀行常務執行役員兼グループIT担当兼ITシステム総括)に加え、銀行業界を代表する関係者が登壇し、知見を共有しました。この対話では、長期的なテクノロジーに関する意思決定、規律ある実行、そしてグローバルなパートナーシップが、新生銀行の進化をどのように形づくってきたのか、またそれらが今後のデジタルロードマップをどのように導き続けているのかが探られました。
ラジーブ・カナン氏(SMBC常務執行役員兼インド事業統括責任者)、ソプネンドゥ・モハンティ氏(Global Finance & Technology Network(GFTN)グループCEO兼シンガポール金融管理局アドバイザー)、ならびにピーター・プランケン氏(GFTNジャパン マネージング・ディレクター兼シンガポール金融管理局アドバイザー)といったグローバルリーダーが出席し、日本がグローバルなフィンテック分野においてますます重要な役割を担っていることについて議論しました。同氏らの見解は、特にAIガバナンス、信頼性の高い金融インフラ、そしてオープンかつセキュアなデジタルエコシステムの将来といった分野において、国境を越えた連携の重要性を示唆しました。
当夜を通じた対話では、規律、精緻さ、そして責任ある運営を基盤として築かれてきた日本の金融機関が、現在、着実にAIを活用したエコシステム主導型の未来へと前進していることが強調されました。これらの対話を通じて、次なる変革の波はテクノロジーだけでなく、グローバルなイノベーションと日本の卓越性へのコミットメントを融合させた、信頼に基づくパートナーシップによって推進されるという共通の信念が、一層強固なものとなりました。
パートナーおよび来賓への感謝の意を表した上で、ヴィシュヌ・R・デュサド氏(ヌークレアス・ソフトウェア共同創業者兼マネージング・ディレクター)は、安定性、セキュリティ、そして長期的な価値を重視して設計されたプラットフォームを通じて、日本を支援し続けるという同社のコミットメントを改めて表明しました。パラグ・ビセ氏(ヌークレアス・ソフトウェアCEO兼エグゼクティブ・ディレクター)は、同社の歩みを振り返り、日本は常に責任あるイノベーションにおけるグローバルなベンチマークであると述べるとともに、世界全体がますますインテリジェント化する中で、機関が自信を持ってスケールできるよう支援するテクノロジーを構築し続けることが、ヌークレアス・ソフトウェアの使命であると語りました。
当夜は、銀行、テクノロジー、そしてフィンテックの各コミュニティを代表するリーダー同士による、温かな意見交換をもって締めくくられました。ヌークレアス・ソフトウェアは、日本における次なる章へと歩みを進めるにあたり、改めて、戦略的パートナーシップを深化させ、日本のデジタル・トランスフォーメーションの目標を支援し、そして信頼性が高くAIに対応し、グローバルに連携したプラットフォームを通じて金融の未来に意義ある貢献を果たすというコミットメントに取り組んでいます。
25年にわたる協業の歩みを礎に、新たなデジタルの可能性が広がる次の時代を見据える中で、ヌークレアス・ソフトウェアは、より強靭で、知的かつ革新的な金融の未来に向かう日本の歩みの一端を担えることを、引き続き光栄に思っています。
本記者発表文の公式バージョンはオリジナル言語版です。翻訳言語版は、読者の便宜を図る目的で提供されたものであり、法的効力を持ちません。翻訳言語版を資料としてご利用になる際には、法的効力を有する唯一のバージョンであるオリジナル言語版と照らし合わせて頂くようお願い致します。
Contacts
For Media related information, please contact:
Deepika Gulabani
Corporate Communications
Email: deepika.gulabani@nucleussoftware.com
Phone: +91-9310334963
FGS Global、2026年版Radarレポート「A Rewired World」を発表 2026年01月09日 11時15分
ニューヨークおよびロンドン--(BUSINESS WIRE)--(ビジネスワイヤ) --FGSグローバルが発表した2026年版Radarレポートによると、戦後の国際秩序は、強権的リーダーシップと取引主義的な関係が多国間協調に取って代わる中で、複数の勢力圏へと分かれつつあります。
FGSグローバルのRadarは、ビジネス、政治、学術、メディアの各界におけるシニア・リーダーおよび政策専門家に対する詳細なインタビュー175件と、米国、カナダ、欧州連合(EU)加盟国、日本の各国の代表性のある約20,000人を対象に行った世論調査をベースとしています。
世界は今、多国間のコンセンサスの崩壊と、弱体化した制度に代わって強権的リーダーシップが台頭することで形作られた「Rewired World」に突入しています。米中競争は貿易、テクノロジー、宇宙の各分野で激化し、従来の同盟には亀裂が生じています。同時に、AI主導のディスラプションが進み、ポピュリスト勢力の台頭が既存の仕組みを揺さぶる中、主要メディアや政党の求心力低下に伴って、影響力は細分化しています。
このように影響力が細分化し、世界が変容する中で、その帰結は、レピュテーションを築き守るうえでの課題と機会の両面が生まれています。
「2026年初頭の国際的な出来事は、世界がいまどのように機能しているかを示す明確なシグナルです」とFGSグローバルのアレックス・ガイザーCEOは語り、「FGSグローバルのRadarレポートは、ボラティリティーとディスラプションが常態でありながら、輪郭がより明確になったこのRewired Worldを進むための不可欠なガイドを、リーダーに提供します」とコメントしています。
「当社の調査によれば、規模や既存企業としての優位性といった従来の強みは急速に揺らいでいます。では、それに取って代わるものは何かというと、競合に先んじて分断された現実を読み取り、民主主義の世論の振れが速まる中で戦略を機動的に切り替え、影響力が細分化したメディア環境で正当性とレピュテーションを積み上げる力です。最も適応力の高い組織は、2026年を乗り切るだけでなく、それを追い風に活用して先行します。」
主な調査結果は以下のとおりです。
調査対象の27か国では、69%が「国際機関よりも世界の強い指導者の方がグローバルな出来事への影響力を増している」と回答しています。
米国、カナダ、欧州、英国、日本では、73%が「次世代の生活は今後より厳しくなる」と考える一方、76%は「自国が分断されていると感じる」と回答しています。
また、74%が「政治のシステムは、一般的な就労者ではなく、富裕層と権力者の利益に資している」と考えています。
本調査は、高所得層とテクノロジー分野が繁栄する一方で低所得層が苦境に陥る「K字型経済」を指摘しています。また、経済が成長しても「恩恵はすでに恵まれている人にしか及ばない」と、回答者の半数が考えています。
市場の乱高下を見込む見方は広く、過半数が「大幅な株式市場の調整が起こる確率は50%を超える」と考え、58%が世界的な金融危機のリスクについて悲観しています。
専門家へのインタビューでは、AIが生産性と効率を高める可能性への楽観が示された一方で、世論は厳格な規制を強く支持しており(83~87%)、68%がAI企業への増税を支持しています。
AIエンジンを信頼する人(34%)は政治家を信頼する人(22%)を上回っており、61%が主流ニュースは信頼できないと回答しています。
FGSグローバルの2026年版Radarレポート「A Rewired World」の全文は、https://fgsglobal.com/radar からダウンロードできます。
編集者向け注記
FGSグローバルRadar 2026について
FGSグローバルRadar 2026は、事業、政治、学術、メディアのシニア・リーダーおよび政策専門家に対する詳細なインタビュー175件に加え、米国、カナダ、EU全加盟国、英国、日本の各国の代表性のある約20,000人を対象にした世論調査をベースとしています。
このインタビューの対象者には、レネ・ハース氏(ARMホールディングスCEO)、エマ・タッカー氏(ウォールストリート・ジャーナル編集局長)、クリスティアン・ゼービング氏(ドイツ銀行CEO)、ローランド・ブッシュ氏(シーメンスAG社長兼CEO)、ダレル・ハケット氏(BMOフィナンシャル・グループCEO)、ショーン・コノリー氏(コナグラ・ブランズ社長兼CEO)、ロドニー・デービス氏(元連邦議会議員・米商業会議所政府渉外責任者)、ティム・デイビー氏(BBC総局長)、デービッド・キャメロン卿(元首相)、ジョナサン・レイノルズ下院議員(大蔵政務次官)、シュリティ・ヴァデラ男爵夫人(プルーデンシャル会長)など、多数の方が含まれています。
発表レポート全文
FGSグローバルの2026年版Radarレポート「A Rewired World」の全文は、https://fgsglobal.com/radar からダウンロードできます。
FGSグローバルについて
FGSグローバルは、戦略コミュニケーションおよびアドバイザリーのリーディングファームとして、事業・政策・評判に関する課題において、複雑なステークホルダー環境に対応するための支援を行っています。
本記者発表文の公式バージョンはオリジナル言語版です。翻訳言語版は、読者の便宜を図る目的で提供されたものであり、法的効力を持ちません。翻訳言語版を資料としてご利用になる際には、法的効力を有する唯一のバージョンであるオリジナル言語版と照らし合わせて頂くようお願い致します。
Contacts
For media inquiries, please contact:
Elizabeth Baker
ebaker@fgsglobal.com
2026年のロボット5大トレンド、国際ロボット連盟が発表 2026年01月08日 12時05分
フランクフルト・アム・マイン--(BUSINESS WIRE)--(ビジネスワイヤ) --産業用ロボット設備の世界の市場価値は、過去最高となる167億ドルにすでに到達しています。数多くの技術革新、市場要因、新たな事業分野により、将来的に需要はさらに高まる見込みです。国際ロボット連盟(IFR)が、2026年に向けてロボット産業における主なトレンド、トップ5を報告いたします。


1 – ロボットにおけるAIと自律化
人工知能(AI)を活用して自律的に作業するロボットの活躍は、ますます広く見られるようになっています。こうした流れにおいてAIがもたらす最大の利点として、ロボットの自律化増大が挙げられます。このトレンドを支えているAIには、いくつかの種類が存在します。分析型AI(Analytical AI)は、大量のデータを処理し、パターンを検出し、実用的な知見をもたらします。このおかげで、たとえばスマートファクトリーでは故障を事前に予測したり、物流の分野では物流経路の計画策定やリソースの配分などをロボットが自律的に行うことが可能となります。
一方、生成AI(Generative AI)は、ルールベースの自動化から、知的で自己進化するシステムへの転換を示唆するものです。生成AIは新しいアウトプットを生み出し、ロボットが新しいタスクを自律的に習得したり、シミュレーションを通じて学習用データを生成したりすることを可能にします。また、これにより、自然言語や視覚情報を用いた指示による人間とロボットの新たな協働が実現します。
ロボットの自律性をさらに発展させる重要なトレンドが、エージェント型AI(Agentic AI)です。この技術は、構造化された意思決定を行う分析型AIと、柔軟性をもたらす生成AIを組み合わせたものです。このハイブリッドなアプローチで、現代のロボットが実世界の複雑な環境においても、より自律性を持って作業するようになることを目指しています。
2 – ITとOTの融合により、ロボットの汎用性が向上
汎用性の高いロボットの需要は急速に拡大しています。直接的な理由として、情報技術(IT)とオペレーショナルテクノロジー(OT)の融合が市場から強く求められてことが挙げられます。ITのデータ処理能力とOTの物理的制御能力を統合することで、リアルタイムのデータ交換、自動化、高度な分析を通じたロボットの汎用性が高まります。この統合は、デジタルエンタープライズとインダストリー4.0の基盤となる要素です。ITとOTの融合により、これまで分断されていた領域の壁が取り払われ、デジタルの世界と物理的な世界の間にシームレスなデータの流れが生まれ、ロボットの能力と汎用性が大幅に強化されます。
3 –信頼性と効率性の実証が鍵となるヒューマノイドロボット
ヒューマノイドロボットの分野は急速に拡大しています。産業用途ヒューマノイドロボットは、特に人間に向けて設計された環境での柔軟性が求められる場面において、有望な技術として注目されています。自動車産業が先駆けとなり、現在、倉庫業務や製造業務における応用が世界的に注目されています。
今日では、企業や研究者たちはすでに試作段階を終え、ヒューマノイドロボットを実環境へ導入する段階へと移行しています。成功の鍵となるのは、信頼性と効率性です。従来の自動化技術と競争するためには、ヒューマノイドロボットがサイクル時間、エネルギー消費、保守コストといった点で、高い産業要件を満たす必要があります。また、産業基準には、工場現場でヒューマノイドロボットに求められる安全レベル、耐久性基準、一貫した性能などが定められています。人手不足を補うことを目的としたヒューマノイドロボットは、人間レベルの器用さと生産性を達成する必要があり、これらは実世界における効率性を証明するための重要な指標となります。
4 – ロボットにおける安全性とセキュリティ
ロボットが工場やサービスの現場で人間と共に作業する機会が増える中、安全な動作の確保はもはや重要であるだけでなく、ロボット産業にとって不可欠な要件となっています。AIによる自律化は安全性の前提を根本的に変えており、試験、検証、人間による監視という作業はより複雑さを増す一方、これまで以上に重要性が高まっています。これは、ヒューマノイドロボットの利用を想定した場合に特に顕著です。ロボットシステムは、ISO安全規格と明確に定義された責任(リスク)分担の枠組みに基づいて設計および認証される必要があります。
ロボットにおけるAIやITとOTの融合という流れの中で、安全性とセキュリティに関してさまざまな課題が生じており、強固なガバナンスと明確な責任の所在が求められています。クラウド接続型、かつAI駆動型の環境へロボットシステムの進出が急速に拡大することで、工業生産は増大するサイバーセキュリティ上の脅威により一層さらされています。専門家たちは、不正なアクセスやシステム操作を可能にする、ロボット制御装置やクラウドプラットフォームを標的としたハッキングの増加を指摘しています。また、ロボットが職場にますます導入されるにつれ、映像、音声、センサーデータなど、ロボットが収集する機密データへの懸念も高まっています。しばしば「ブラックボックス」と呼ばれるディープラーニングモデルは、開発者自身でさえ説明するのが困難、あるいは不可能な結果を生み出すことがあります。こうした責任の所在をめぐる法的および倫理的な不透明さを背景に、AIの導入を統治するための明確な枠組みを求める声が高まっています。
5 – 労働力不足に取り組む「味方」としてのロボット
世界中の雇用主が、必要とされる専門的スキルを持つ人材の確保に苦慮しています。こうした人材不足は、既存の従業員が追加のシフトを担うことにつながり、あらゆる業界でストレスや疲労の増大を招いています。この課題に対処するための重要な戦略の一つが、ロボットと自動化の導入です。この変革プロセスにおいて、雇用主は人間の労働力を巻き込むことで大きなメリットを得ることができます。ロボットの導入において従業員との密接な協力を図ることは、受容性を確保する上で極めて重要です。このことは、工業生産の現場だけでなく、さまざまなサービス分野においても同様です。労働力不足への対応、単純作業の代替、あるいは新たなキャリア機会の創出といったロボットがもたらす利点により、ロボットは職場で協働するパートナーとして受け入れられるでしょう。同時に、ロボットは若者にとって職場をより魅力的なものにする一つの手段ともなります。企業や政府は、変わりゆくスキルの需要に労働者が対応し、自動化主導の経済において競争力を維持できるよう、スキルの習得や再教育のプログラムを推進しています。
ダウンロード
英語版とドイツ語版のプレスリリース、ならびに画像は以下からダウンロードできます。 https://ifr.org/ifr-press-releases/
IFRについて 国際ロボット連盟(IFR)は世界のロボット産業に関する情報を発信しており、世界20カ国以上の ロボット協会(全国組織)、学術団体、産業用ロボット・サービスロボットの製造業界を代表して います。www.ifr.org
Contacts
報道機関向けお問い合わせ先
国際ロボット連盟(International Federation of Robotics)
広報担当(PRESS OFFICER)
カルステン・ヘール(Carsten Heer)
電話:+49 (0) 40 822 44 284
メール:press@ifr.org
ハイトマン、 福岡県の物流拠点「鳥栖セントラルディストリビュ… 2026年01月08日 09時20分
この取引は、福岡における単一資産の物流施設取得としては過去最大規模となり、九州がアジアの主要な地域投資市場として台頭していることを示している
東京--(BUSINESS WIRE)--(ビジネスワイヤ) --グローバル不動産投資顧問会社のハイトマンLLC(以下「ハイトマン」)は、九州の主要物流拠点の1つに位置する鳥栖セントラルディストリビューションセンター(以下「鳥栖セントラルDC」)の取得を発表しました。この取引は、福岡地域における単一資産の物流取引としては過去最大規模となります。この投資は、ハイトマンが運用するファンドと、ハイトマンの戦略的資本パートナーである三菱HCキャピタルリアルティ株式会社(以下「三菱HCキャピタルリアルティ」)および三井住友ファイナンス&リース株式会社の戦略子会社であるSMFLみらいパートナーズ株式会社(以下「SMFLみらいパートナーズ」)を代表して、ハイトマンにより実行されました。


この最先端施設は、北棟と南棟を合わせて1万7676坪(62万8968平方フィート)の賃貸スペースで構成されています。最適な効率性を実現するよう設計されたこの施設には、待ち時間を短縮するために横並びで積み込みができる柱のないトラックバースが備わっています。この施設は、年間440万kWh以上の発電が可能と見込まれる6200枚以上の太陽光パネルを含む、持続可能性の特徴を備えており、CASBEE評価でA認証を取得しています。
今回の取得は、ハイトマンが誇る不動産ソーシングと価値創造に関する専門性、そして日本の三菱HCキャピタルリアルティとSMFLみらいパートナーズが持つ株式および債券資本市場における能力が一つとなって実現したものです。
ハイトマンのアジア太平洋プライベート・エクイティ・グループのマネージング・ディレクター兼共同責任者であるブラッド・フー氏は次のように述べています。「鳥栖は、福岡から九州全域にわたる高度な製造業の成長と流通活動の中心地です。鳥栖セントラルディストリビューションセンターは、この地域で事業を拡大し続けている幅広いユーザーのニーズに応えることができると考えています。特に、日本市場における大手投資家である三菱HCキャピタルリアルティとSMFLみらいパートナーズとの資本提携は、福岡地域の今後の力強い成長が見込まれるという当社の見通しを裏付けるものであり、大変嬉しく思います」
九州自動車道、長崎自動車道、大分自動車道の交差点である鳥栖インターチェンジから2.3kmという戦略的な場所に位置する鳥栖セントラルDCは、九州全域への直結アクセスを実現します。また、甘木鉄道に近いことから、従業員のアクセスも容易で、テナント運営をサポートするという利点もあります。
三菱HCキャピタルリアルティは、「鳥栖セントラルディストリビューションセンターは、九州全域をカバーする戦略的な立地にあり、近年半導体などの先端技術の拠点となっているこの地域の多様な物流ニーズを取り込むのに有利な立場にあります。今回の投資を通じて、地域の産業と経済の発展に貢献できることを光栄に思います」と述べています。
SMFLみらいパートナーズは、ハイトマンや三菱HCキャピタルリアルティといったグローバルでの経験豊富なパートナーと共に、高品質な物流施設の提供を通じて日本の物流インフラの発展を支援し、地域経済と地域社会の持続的な成長に貢献してまいります。
鳥栖市場は、過去4年間で6兆円を超える設備投資を記録した半導体製造業の成長を背景に、九州地域の中核物流拠点へと急速に発展しました。福岡は、堅固なインフラと人口流入に支えられ、日本で最も急速に成長している都市の一つであり続けています。
ハイトマンは、物流施設、住宅、セルフストレージ、オフィスなど、日本の主要不動産セクターにおいてポートフォリオを拡充し続けています。ハイトマンは、今回の取得に加え、福岡において9件の住宅ポートフォリオの取得を完了し、日本市場へのコミットメントをさらに強化しました。
ハイトマンについて:
ハイトマンは、2025年9月30日現在で480億ドルの資産を運用するグローバル不動産投資顧問会社です。1966年に設立し、シカゴにグローバル本社を、ロンドンに欧州本社を置くハイトマンは、世界10か所にオフィスを構え、世界の不動産市場と資本市場において、積極的な活動を展開しています。ハイトマンは、プライベート・エクイティ、債券、上場不動産投資信託を通じて不動産投資を行っています。
三菱HCキャピタルリアルティについて:
三菱HCキャピタルリアルティは、投資した不動産のバリューアップを図る不動産再生投資事業のほか、不動産ソリューション事業、物流投資事業、ホテル投資事業、不動産ファイナンス事業など、不動産の価値を高めるさまざまな取り組みを通じて、都市や社会の課題解決に取り組んでいます。
三菱HCキャピタルリアルティウェブサイト:https://www.mitsubishi-hc-capital-realty.co.jp/english/
SMFLみらいパートナーズについて:
SMFLみらいパートナーズは、金融機能を持つ事業会社として、不動産事業を主力事業の一つに位置付けております。不動産リース、ノンリコースローン、エクイティ出資などの幅広いソリューションの提供を通じて、多岐にわたる分野でお客さまやビジネスパートナーと共に、新たな価値を共創しています。今後も、賃貸住宅、物流施設、オフィス、商業施設等への投融資を通じて、地域経済および地域社会の持続的な発展に貢献していきます。
SMFLみらいパートナーズウェブサイト:https://www.smfl-mp.co.jp/
本記者発表文の公式バージョンはオリジナル言語版です。翻訳言語版は、読者の便宜を図る目的で提供されたものであり、法的効力を持ちません。翻訳言語版を資料としてご利用になる際には、法的効力を有する唯一のバージョンであるオリジナル言語版と照らし合わせて頂くようお願い致します。
Contacts
Prosek Partners on behalf of Heitman
pro-Heitman@prosek.com
ハイトマン、福岡市で9棟の住宅ポートフォリオを取得 2026年01月08日 09時16分
福岡市における史上最大規模のマルチアセット住宅ポートフォリオ取得、日本国内で最も成長著しい住宅市場と確信
東京--(BUSINESS WIRE)--(ビジネスワイヤ) --グローバル不動産投資顧問会社であるハイトマンLLC(以下「ハイトマン」)は、福岡市における9棟からなる住宅ポートフォリオの取得を発表しました。本投資は、グローバル・コアプラス戦略を進めるハイトマン、およびハイトマンの戦略的資本パートナーであり三井住友ファイナンス&リース株式会社の戦略子会社であるSMFLみらいパートナーズ株式会社(以下「SMFLみらいパートナーズ」)により実施されました。本取引は、同市における史上最大規模のマルチアセット住宅ポートフォリオ取得となります。


「当社は福岡市場において投資規模の拡大に戦略的に注力してきました」と、ハイトマンのアジア太平洋アクイジション部門のマネージング・ディレクター兼責任者であるブラッド・フーは述べています。「福岡都市圏内の他セクターで完了した取引に加えて、本ポートフォリオは、良好な人口動態、適正住宅費負担(アフォーダビリティ)、そして大規模な産業投資流入に支えられたこの重要な市場における当社の長期的なポジション構築に貢献するものです」
本ポートフォリオは、博多や平尾など福岡市内の主要サブマーケットに分散配置された316戸で構成されています。全物件から福岡市の天神・博多ビジネス地区へ、地下鉄やバスの直通路線を利用して15分以内でアクセスできます。物件としてはスタジオタイプ、1ベッドルーム、2ベッドルーム、3ベッドルームのユニットなど多様な構成で幅広い入居者層に対応しており、活動的な若手プロフェッショナル層向けから家族の増加を見据えたファミリー向けの間取りまでさまざま揃えています。
SMFLみらいパートナーズは、お客様やビジネス・パートナーの皆様と共に、高品質な住宅供給を通じて、安定した人口流入と雇用に支えられた福岡市の社会インフラを支え、長期的な魅力を高めることに貢献していきます。
ハイトマンは市場外取引により本ポートフォリオを取得しましたが、これは賃料回復力と長期的な市場賃料上昇可能性を兼ね備えた魅力的なエントリー・ポイントとなりました。福岡は釜山や上海などアジアの主要拠点都市に近接していることから、日本への新規移住者にとって国際的な魅力がますます高まっています。
福岡市は日本の主要都市の中で最も高い人口増加率を維持しており、この増加傾向は2040年まで続くことが予測されています。ハイトマンの住宅ポートフォリオ取得は、九州における最近の主要物流センター買収と連動するもので、福岡都市圏市場における過去最大規模の単一資産物流取引でもあります。
ハイトマンについて:
ハイトマンはグローバルな不動産投資顧問会社であり、2025年9月30日現在で運用資産は480億ドルに達しています。1966年に設立し、グローバル本社をシカゴに、欧州本社をロンドンに置き、世界11都市にオフィスを構え、世界の不動産市場や資本市場において積極的な投資活動を展開しています。ハイトマンは、プライベート・エクイティ、債券、公開不動産証券を通じて不動産投資を行っています。
SMFLみらいパートナーズについて:
SMFLみらいパートナーズは、金融機能を持つ事業会社として、不動産事業を主力事業の一つと位置付けています。不動産リース、ノンリコースローン、エクイティ出資など幅広いソリューションの提供を通じて、多岐にわたる分野にてお客様やビジネスパートナーと、新たな価値を共創しています。今後も、住宅用不動産、物流施設、オフィス、商業施設等への投融資を通じて、地域経済および地域社会の持続可能な発展に貢献していきます。
本記者発表文の公式バージョンはオリジナル言語版です。翻訳言語版は、読者の便宜を図る目的で提供されたものであり、法的効力を持ちません。翻訳言語版を資料としてご利用になる際には、法的効力を有する唯一のバージョンであるオリジナル言語版と照らし合わせて頂くようお願い致します。
Contacts
Prosek Partners on behalf of Heitman
pro-Heitman@prosek.com
キオクシア:クライアントPC向けKIOXIA BG7シリーズSSDを発表 2026年01月06日 14時36分
第8世代BiCS FLASH™ 3次元フラッシュメモリをクライアント向けSSDで初めて採用
東京--(BUSINESS WIRE)--(ビジネスワイヤ) --キオクシア株式会社(以下、キオクシア)は、CMOS Directly Bonded to Array (CBA)技術を活用する第8世代BiCS FLASH™3次元フラッシュメモリ(1)を採用したクライアントSSD「KIOXIA BG7シリーズ」(以下「BG7シリーズ」)を発表し、一部のPC OEM顧客向けにサンプルの提供を開始しました。BG7シリーズSSDは、2026年1月6日から8日までラスベガスにて開催される CES® (Consumer Electronics Show)で展示されます。
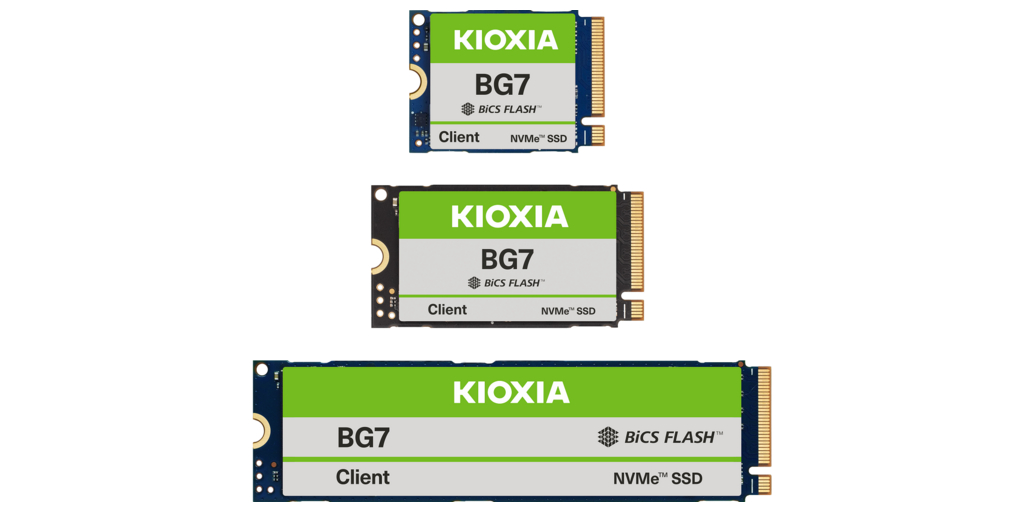



BG7シリーズは第8世代BiCS FLASH™フラッシュメモリの優れた性能と消費電力効率を、幅広いアプリケーションに向けて提供します。シーケンシャルリード性能は最大7,000 MB/s、ランダムリードおよびライト性能は最大1,000,000 IOPSの高性能を実現し、既存のKIOXIA BG6シリーズと比較して、それぞれ約16%および10%性能を向上しました。また、CBA技術によって向上したメモリセルパフォーマンス、効率の良い制御回路、コントローラーなどのSSD側の性能向上により、シーケンシャルライト性能における電力効率は約67%改善しています。
コストパフォーマンスを重視したDRAM非搭載のクライアントSSDであるBG7シリーズは、ホスト側のメモリの一部を利用するホストメモリバッファ (HMB) 技術をサポートします。フォームファクターは既存のM.2 Type 2230およびType 2280に加え、新たにType 2242モデルが追加され、幅広いアプリケーションや搭載条件に対応しています。
KIOXIA BG7シリーズの他の主な特長:
PCIe® 4.0、NVMe™ 2.0d準拠
TCG Opalバージョン2.01による自己暗号化ドライブ(SED)サポート
容量ラインアップ: 256 GB、512 GB、1024 GB、2048 GB
注釈:
(1) KIOXIA BG7シリーズ256GBモデルは第6世代BiCS FLASH™フラッシュメモリ を採用
記憶容量:1 MB (1メガバイト) =1,000,000 (10の6乗) バイト、1 GB (1ギガバイト) =1,000,000,000 (10の9乗) バイト、1 TB (1テラバイト) =1,000,000,000,000 (10の12乗) バイトによる算出値です。しかし、1 GB=1,073,741,824 (2の30乗) バイト、1TB=1,099,511,627,776 (2の40乗) バイトによる算出値をドライブ容量として用いるコンピューターオペレーティングシステムでは、記載よりも少ない容量がドライブ容量として表示されます。ドライブ容量は、ファイルサイズ、フォーマット、セッティング、ソフトウェア、オペレーティングシステムおよびその他の要因で変わります。
読み出しおよび書き込み速度は、ホストシステム、読み書き条件、ファイルサイズなどによって変化します。
IOPS: Input Output Per Second (1秒間に読み書きできる回数)
CES® は the Consumer Technology Association の登録商標です。
NVMeは、NVM Express, Inc.の米国またはその他の国における登録商標または商標です。
PCIeはPCI-SIGの商標です。
その他記載されている社名・商品名・サービス名などは、それぞれ各社が商標として使用している場合があります。
*本資料に掲載されている情報(製品の価格/仕様、サービスの内容およびお問い合わせ先など)は、発表日現在の情報です。予告なしに変更されることがありますので、あらかじめご了承ください。
Contacts
報道関係の本資料に関するお問い合わせ先:
キオクシア株式会社
プロモーションマネジメント部
進藤智士
Tel: 03-6478-2404
SBCメディカルグループ、米国大手メディスパ・プラットフォーム… 2026年01月06日
カリフォルニア州アーバイン--(BUSINESS WIRE)--(ビジネスワイヤ) -- 国内外の医療機関に経営支援を行う SBC メディカルグループホールディングス(所在地:米国カリフォルニア州、CEO:相川 佳之、以下「SBC」)は、本日、米国を拠点とする大手メディスパ・プラットフォーム、OrangeTwist社への戦略的少数持分投資を完了するとともに、同社との体系的な協業フレームワークを構築したことを発表しました。本取引は、当社の長期的な機関投資家であるHildred CapitalおよびAthyrium Capitalと連携して実施されています。本件は、美容医療分野における最重要市場の一つである米国へのSBCメディカルグループの正式参入を意味すると同時に、当社の中長期的なグローバル成長戦略を具体的に前進させる重要なマイルストーンです。詳細資料はこちら。



SBCは国内外に258のクリニックネットワークを擁し、その年間総来院数は600万人を超えます。25年以上にわたり、多様な医療分野で蓄積してきた臨床知見、拡張性の高い運営モデル、計画的な成長実績を統合し、世界最大級かつ高度に洗練された医療プラットフォームを構築してきました。これらの基盤は、次なる国際展開フェーズを実行するうえでの競争優位性を支えるものです。
2015年設立のOrangeTwist社は、非侵襲的な美容医療施術を専門とし、現在、米国6州において24拠点を展開しています。強固な医療ガバナンスのもと、データに基づいた臨床運営体制を構築し、注入治療、レーザー治療、再生医療など、幅広い施術を提供しています。また、調達、臨床ワークフロー、リアルタイムでのKPI管理を一体的に運用する高度なマネジメントシステムにより、運営の一貫性を確保するとともに、事業拡大に対応可能な成長基盤を構築しています。本投資を起点として、SBCメディカルグループはOrangeTwist社との強固なパートナーシップのもと、米国市場における本格的な戦略展開を開始します。米国とアジアにおける両社の強みを相互に活用し、中長期的な成長機会の創出を図ります。
SBCメディカルグループ CEO 相川佳之のコメント
OrangeTwist社は、非侵襲的美容医療分野において、卓越した品質と優れた顧客体験を一貫して提供することで、米国市場におけるリーダーシップを確立してきました。同社の強みと、当社が有する専門性およびグローバルネットワークを融合させることで、アジアを含むグローバル市場における成長をさらに加速できるものと考えています。
OrangeTwist 共同創業者 クリント・カーネル氏のコメント
SBCメディカルグループは、国際的に高い評価を受ける、極めて洗練された美容医療プラットフォームを構築してきました。OrangeTwistとのパートナーシップを通じた同社の米国参入は、他に類を見ない強固なオペレーション基盤をもたらし、世界最大かつ最も成長著しい美容医療市場におけるスケール拡大を大きく前進させるものです。
SBCメディカルグループのグローバル成長戦略
OrangeTwist社への投資は、米国の医療美容およびウェルネス市場において持続的な競争優位の確立を目指す、SBCメディカルグループの段階的な中長期成長戦略における第一段階です。本提携を通じ、臨床プロトコル、テクノロジー導入、オペレーション効率、事業戦略といった分野において、米国およびアジアそれぞれで培ってきた知見や運営ノウハウを相互に活用することが可能となります。本パートナーシップは、「フェーズ1:参入」戦略の開始を示すものであり、将来的な事業拡張および市場におけるプレゼンス拡大に向けた基盤を形成します。SBCメディカルグループは、個人のウェルビーイングと高品質な医療成果を重視した持続可能なモデルを統合することで、米国およびアジアにおいて独自の価値提供を行い、地域分散型で安定性と成長性を兼ね備えた収益基盤の構築を目指します。
● 持続的な収益成長を志向したグローバル展開方針
SBCメディカルグループは、特に米国および東南アジアへの展開を、長期的な企業価値創造の中核と位置づけています。当社のグローバル展開方針は、以下の3原則に基づいています。
高い実行力を有する現地パートナーとの提携
強固なブランド力、拡張性の高いビジネスモデルを有する地域オペレーターとの戦略的提携
当社の差別化された運営ノウハウの展開
先進的な美容医療、卓越したオペレーション管理、臨床安全性におけるSBCメディカルグループの差別化された運営ノウハウの展開によるパフォーマンス向上
独自プラットフォームおよび最先端のLongevity技術への早期参入による市場優位性の確立
最先端の医療技術、独自の治療プラットフォーム、情報共有基盤への早期参入を通じた市場優位性の確保と、将来的なイノベーションおよび利益率拡大
当社は、再生医療、美容医療、ウェルネス医療といった高成長分野に注力するとともに、人間の最適化分野(Longevity、Medicine 4.0など)といった次世代領域についても視野に入れています。さらに、AIを活用した診断技術や遠隔医療、患者体験の向上に資する技術など、長期的な成長戦略と整合する医療技術基盤の構築を推進しています。
● グローバル事業戦略
フェーズ1:参入(2025–2026年)
市場理解を深化させ、戦略的パートナーシップを構築し、将来の拡張に向けた選択肢を確保するマイノリティ投資の実行や、クリニックの開院を目指す
フェーズ2:拡大(2027–2028年)
蓄積された市場知見と確立されたオペレーションを活用し、成長加速。競争優位性の強化につながる戦略的なM&Aおよびジョイントベンチャーを推進
フェーズ3:リーダーシップ(2029年以降)
多角化された収益基盤、拡張性の高い医療プラットフォーム、テクノロジー主導のオペレーションの効率化を背景に、米国および重点グローバル市場におけるリーディングカンパニーとしての地位を確立
SBCメディカルグループは、日本事業の安定性と収益性を基盤に、この規律ある3フェーズのグローバル戦略アプローチの両輪によって、持続的な長期成長、収益力の質的向上、ならびにグローバルブランド価値の継続的な拡大を実現してまいります。
SBC メディカルグループホールディングス
SBC メディカルグループホールディングスは、先進的な美容医療をはじめ、皮膚科、整形外科、不妊治療、歯科、AGA、眼科など幅広い診療領域のフランチャイズ経営を行う総合医療グループです。多様なクリニックブランドを擁し、メディカルツーリズムや米国・アジアへのグローバル展開も進めています。2024 年9 月に米国 NASDAQ 市場へ上場し、2025 年 6 月には米国株価指数「ラッセル 3000」にも選出されました。今後も「メディカルイノベーションで世界中の人々の幸福度向上に貢献する」というグループパーパスの実現に向け、信頼性の高い医療サービスの提供とネットワーク拡大を推進してまいります。
英文名:SBC Medical Group Holdings Incorporated
上場市場:NASDAQ Global Market
ティッカー(米国証券コード):SBC
所在地:200 Spectrum Center Drive Suite 300 Irvine, CA 92618 USA
CEO:相川 佳之
事業:医療機関(総合美容医療・皮膚科・歯科・AGA 治療・婦人科・不妊治療・眼科・整形外科・
再生医療、他)への経営支援事業
公式ウェブサイト:https://sbc-holdings.com/jp
公式LinkedInページ:https://www.linkedin.com/company/sbc-medical-group-holdings-inc
OrangeTwist社について
OrangeTwist は、医師の監督のもとで美容医療およびウェルネス治療を提供する、全米規模で急成長中の事業者です。現在、カリフォルニア州、テキサス州、ワシントン州、ネバダ州、コロラド州、ニュージャージー州の 6 州において、24 拠点を展開しています。同社は、世界的に著名なメディカル・ディレクターである Dr. Grant Stevens, M.D., F.A.C.S. と、ヘルスケアサービス/医療テクノロジー分野において豊富な経験を有し、HydraFacial Company の元 CEO を務めた Clint Carnell によって共同設立されました。OrangeTwist は、注入治療、エネルギー系治療、再生医療系治療からなる厳選されたポートフォリオを、最高水準の臨床チームと強固な医療ガバナンス体制に支えられた、ホスピタリティを重視した体験を通じて提供しています。詳細については、www.orangetwist.com をご覧ください。
Hildred社について
Hildred は、ヘルスケア分野に特化したプライベート・エクイティ・ファームであり、ミドルマーケット企業における価値創出の機会を追求しています。経営陣とのパートナーシップを重視し、プラットフォームの拡張、収益成長の創出、戦略的およびオペレーション面での改善、事業開発の推進、ならびにバリュエーションのマルチプル拡大を通じて、企業価値の向上を支援することを専門としています。詳細は www.hildred.com をご覧いただくとともに、LinkedIn にて同社をフォローしてください。
Athyrium社について
Athyrium は、2008 年に設立された、グローバルなヘルスケア分野における投資機会に注力する専門的な資産運用会社です。46億ドル超のコミットメントキャピタルを有するファンドに対し、投資助言を行っています。同社のチームは、上場株式、プライベート・エクイティ、債券、ロイヤルティ、その他のストラクチャード・セキュリティなど、幅広い資産クラスにわたる豊富な投資経験を有しています。また、バイオ医薬品、医療機器・医療製品、ヘルスケア関連サービス、ヘルスケア情報技術を含む、あらゆるヘルスケア分野に投資を行っています。詳細については、www.athyrium.com をご覧ください。
将来の見通しに関する記述
本プレスリリースには、将来の見通しに関する記述が含まれています。将来の見通しに関する記述は、過去の事実や現在の状況に関する記述ではなく、将来の出来事や業績に関する当社の見解のみを示すものです。将来の出来事や業績の多くは、その性質上、本質的に不確実であり、当社のコントロールの及ばないものです。これらの将来見通しに関する記述は、とりわけ、当社による OrangeTwist への戦略的投資および提携、当社の製品ローンチ計画および戦略、売上高および利益の成長、ならびに事業の見通しに関する当社の現時点での見解を反映したものです。場合によっては、「可能性がある」、「はずである」、「期待する」、「予想する」、「企図する」、「推定する」、「考える」、「計画する」、「予測する」、「予測する」、「可能性がある」、「希望する」といった言葉や、これらの否定語または類似語の使用により、将来の見通しに関する記述を特定することができます。当社は、本リリースの日付時点においてのみ最新であり、様々なリスク、不確実性、仮定、または予測や定量化が困難な状況の変化の影響を受ける将来見通しに関する記述を過度に信頼しないよう注意を促します。将来の見通しに関する記述は、経営陣の現在の予想に基づくものであり、将来の業績を保証するものではありません。当社は、法律で義務付けられている場合を除き、将来予想に関する記述の予想の変更、または当該記述の根拠となる事象、条件、状況の変化を反映するために、将来予想に関する記述の更新または修正を公に発表する義務を負うものではありません。そのような要因には、特に、世界的、地域的、または地方的な経済、事業、競争、市場、規制の状況の変化、および米国証券取引委員会(SEC)のウェブサイト(www.sec.gov)からアクセス可能な、当社が米国証券取引委員会(SEC)に提出した書類の「リスク要因」の見出しおよびその他の箇所に記載されているものが含まれます。
Contacts
福井 輝 / IR部長 E-mail: ir@sbc-holdings.com
脇山 亜希子 / CPRO E-mail: pr@sbc.or.jp

